
A triply charged ion with velocity 7.00 × 10 6 7.00 × 10 6 m/s.What is the worst case of response to a credit card security breach.
An ion has a mass of 8.29 × 10-27 kg and a charge of +4e.A 0.5 T magnetic field is measured at the center of a 10-turn.paid an annual dividend of $2.10 yesterday.You are the CFO of a soon-to-be Multi-National Corporation.The correct ranking cannot be determined. Rank from highest to lowest melting point. Rank from highest to lowest melting.Īrrange the following hydrocarbons in order of decreasing melting point. Arrange the following hydrocarbons in order of decreasing melting point.Reset Help triethylamine di n-propylamine cyclohexylamine Highest Lowest To rank items as equivalent, overlap them. Rank the following compounds from compounds with highest boiling point to compounds with lowest boiling point. Rank the following compounds in order of decreasing boiling point (lowest to highest). Rank the following compounds in order of decreasing boiling point (lowest to highest).

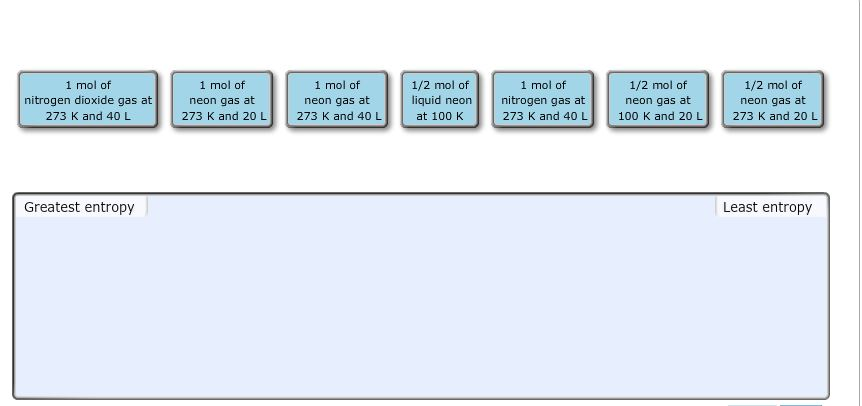
► View Available Hint(s) Reset Help 1 mol of oxygen gas at 273 K and 40 L 1 mol of nitrogen dioxide gas at 273 K and 40 L 1/2 mol of liquid helium at 100 K 1/2 mol of helium gas at 100 K and 20 L 1/2 mol of helium gas at 273. Qualitative Predictions about Entropy Rank these systems in order of decreasing entropy.

Rank these systems in order of decreasing entropy Rank from highest to lowest entropy. Rank these systems in order of decreasing entropy Rank from highest to lowest entropy.View Available Hint(s) Reset Help Greatest entropy Least entropy 1 malat nitrogen gas at 273 K and 40L 1 mol of radon gas at 273 K and 40 L 1 molo hydrogen peroxide ges at 273 K and 40 L 1 malo radon gas at 273 K and 20 L 1/2 malo redon gesat 273 K and. Part A Rank these systems in order of decreasing entropy.L1/2 mol of liquid xenon at 100 K1/2 mol of xenon gas at 100. K and 20 L1 mol of hydrogen peroxide gas at 273 K and 40 L1 mol ofįluorine gas at 273 K and 40 L1 mol of xenon gas at 273 K and 20 View Available Hint(s) ResetHelp 1/2 mol of xenon gas at 273
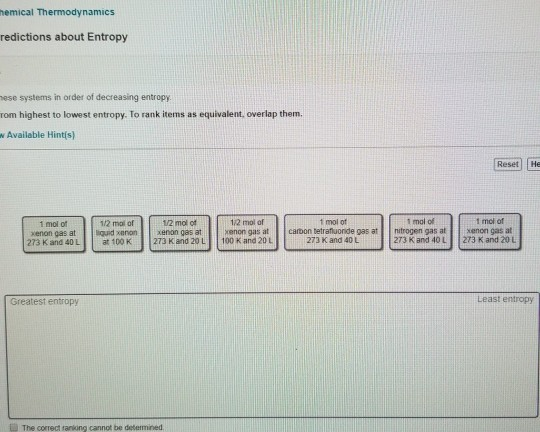
Rank these systems in order of decreasing entropy.


 0 kommentar(er)
0 kommentar(er)
